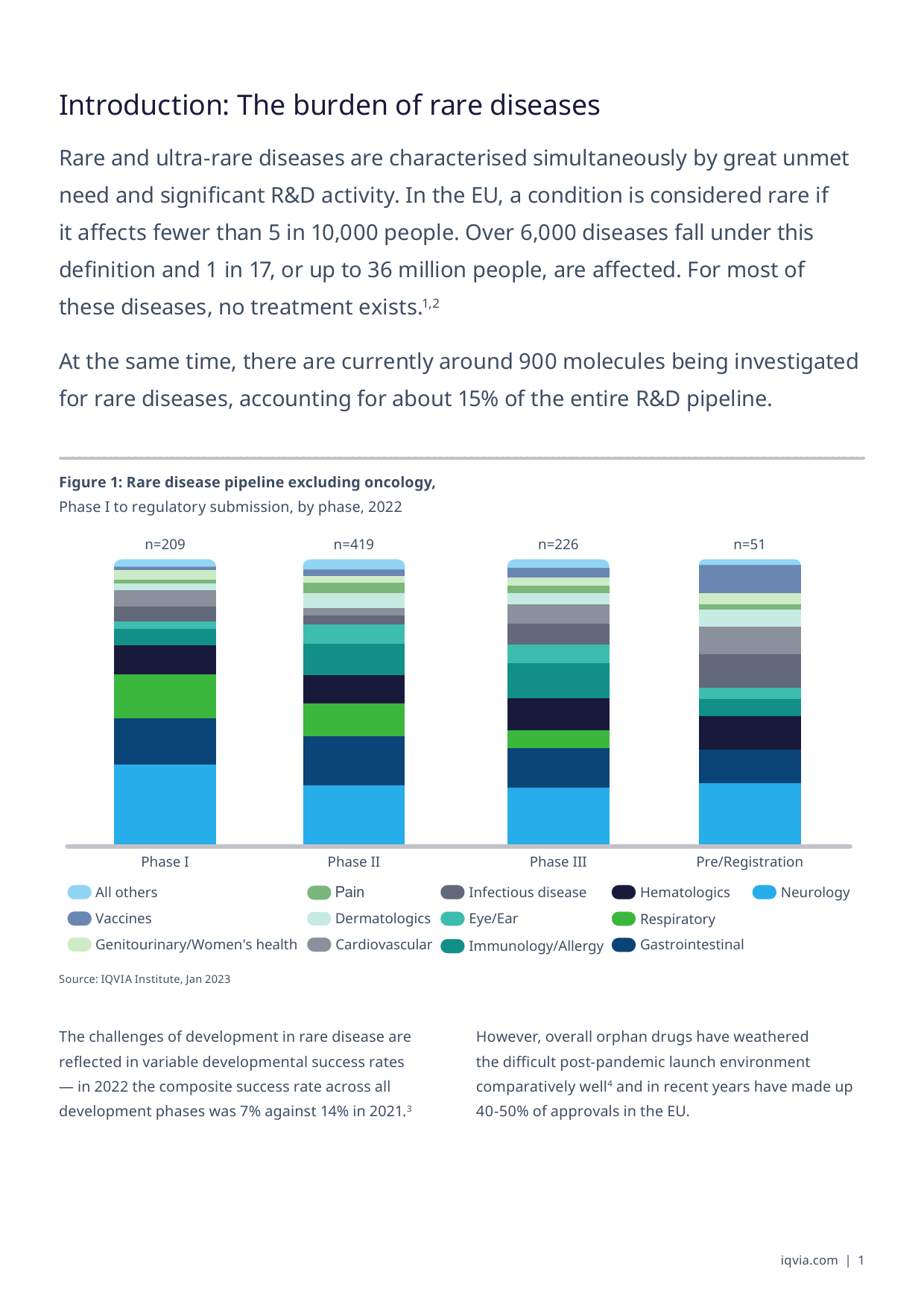
WhitePaperHarnessingthePowerofPatientOrganisations:ACaseStudyofPO-DrivenDrugDevelopmentinUltra-RareDiseaseMEIKEMADELUNG,EngagementManager,EMEAThoughtLeadership,IQVIAHELENABAYLEY,Analyst,EMEAThoughtLeadership,IQVIATableofcontentsIntroduction:Theburdenofrarediseases1DevelopingatreatmentforAlkaptonuria(AKU)2WhatisAlkaptonuria?2Identifyingapotentialtreatmentcandidate2Establishingaresearchconsortium3Trialplanning,design,andexecution5Patientidentificationandrecruitment5Enablingpatientretentionandprovidingsupport6Lessonslearnedandfutureoutlook7Successfactors7Lookingahead:towardsacureforAKU8Recommendationsforcreatingasuccessfulcollaborationbetweenpatientorganisationsandpharma8References11Abouttheauthors13Introduction:TheburdenofrarediseasesRareandultra-rarediseasesarecharacterisedsimultaneouslybygreatunmetneedandsignificantR&Dactivity.IntheEU,aconditionisconsideredrareifitaffectsfewerthan5in10,000people.Over6,000diseasesfallunderthisdefinitionand1in17,orupto36millionpeople,areaffected.Formostofthesediseases,notreatmentexists.1,2Atthesametime,therearecurrentlyaround900moleculesbeinginvestigatedforrarediseases,accountingforabout15%oftheentireR&Dpipeline.Figure1:Rarediseasepipelineexcludingoncology,PhaseItoregulatorysubmission,byphase,2022n=209n=419n=226n=51PhaseIPhaseIIPhaseIIIPre/RegistrationPhaseIPhaseIIPhaseIIIPre/R...


发表评论取消回复