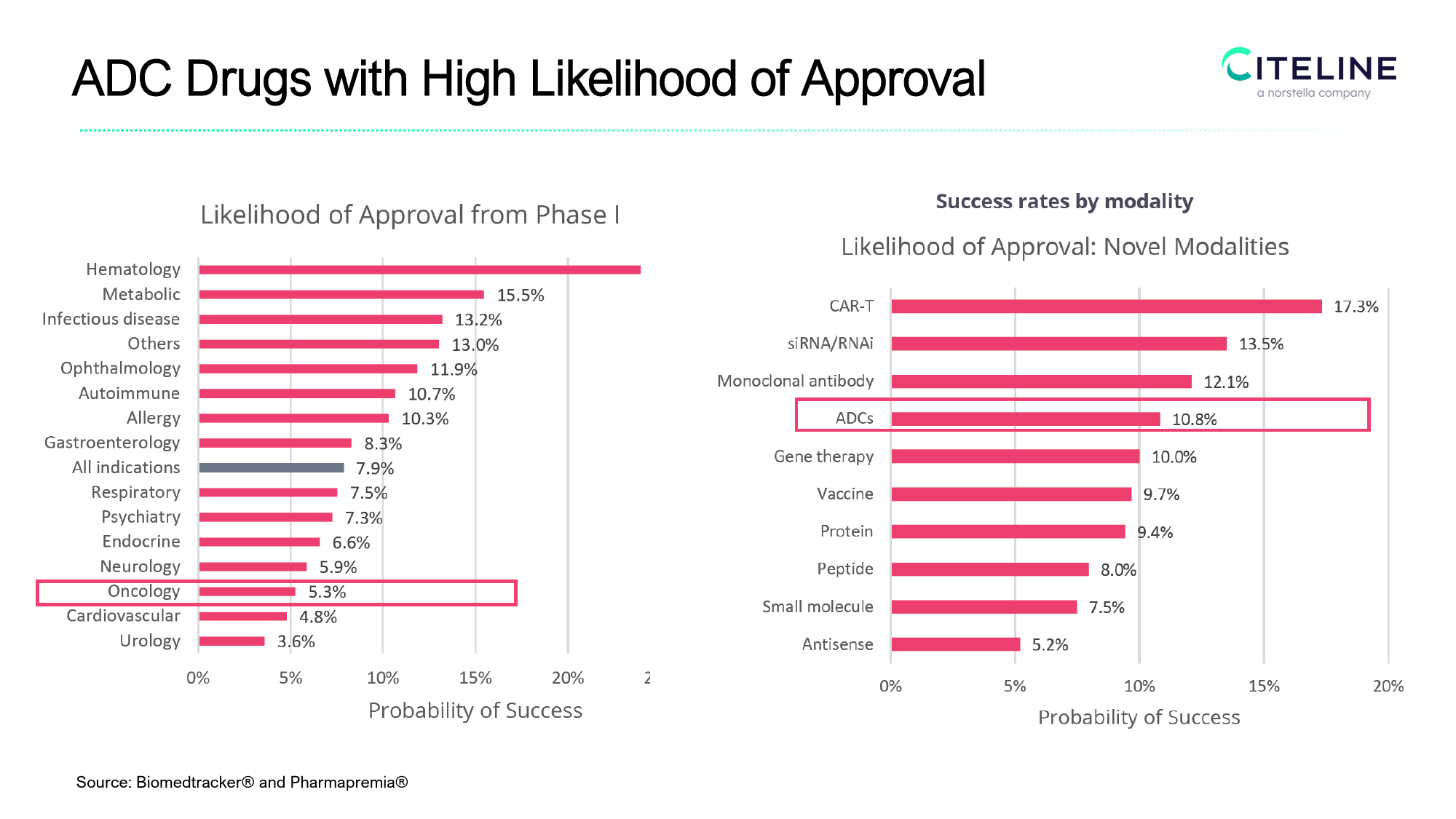
ADCClinicalTrialStrategyShuhuaZhou(周淑华)PhDCitelineMar.9th2024Outline1.ADC领域的市场空间和临床试验启动趋势2.临床可行性评估(患者数量,临床时长,Patientjourney)3.ADC临床开发的适应症和患者、主要终点选择4.ADC临床试验的Combo趋势ADCDrugswithHighLikelihoodofApprovalSource:Biomedtracker®andPharmapremia®ADCDrugsWWsalesforecastWWSalesbyIndication($m)IndicationMarketShareChange/2028(m$)CAGR26.9%1.00%31,227.535,000.0Breastcancer30,000.026,516.625,000.021,934.30.80%20,000.017,501.4Indicationsales(m$)0.60%IndicationMarketShare15,000.013,585.10.40%Bladdercancer0.20%10,000.07,469.810,118.3Non-smallcelllungcancer(NSCLC)Non-Hodgkinlymphoma(NHL)HodgkinlymphomaOvariancancer5,000.00.00%-2000-10000100020003000400050006000700080000.0202320242025202620272028-0.20%SalesChangeOverTime2022Source:EvaluatePharma[Accessed2024]ADCDrugsTrialsbyStartDate250200TrialNumber150IVIII/IV100IIIII/IIIII50I/III01995199719981999200020012002200320042005200620072008200920102011201220132014201520162017201820192020202120222023Source:TrialtroveOutline1.ADC领域的市场空间和临床试验启动趋势2.临床可行性评估(Patientjourney,患者数量,临床时长与成本)3.ADC临床开发的适应症和患者、主要终点选择4.ADC临床试验的Combo趋势PatientDemographicsandPatientJourney•Betterunderstandingofpatientdynamicstoassistwithpharmacompanystrategicplanning–improveclinicaltrialdes...


发表评论取消回复